Health Canada (HC) and Food Standards Australia New Zealand (FSANZ) have implemented a collaborative arrangement regarding the pre-market assessment of GM foods: the HC-FSANZ Shared Assessment Process.
The following information is intended for applicants interested in submitting a product to be assessed and authorised for food use in Canada, Australia, and New Zealand, through the Health Canada-FSANZ Shared Assessment Process.
The Shared Assessment Process
The Shared Assessment Process is a collaborative arrangement between Health Canada and FSANZ whereby an application for the pre-market assessment of a genetically modified product for food use (i.e., a GM food1) may be submitted to both agencies, but only one food safety assessment is prepared (either by Health Canada or FSANZ). The assessment is then referred to the other agency for review and input to ensure it meets the requirements of both agencies.
The shared assessment is then used by both Health Canada and FSANZ for their own separate and independent regulatory decision regarding the GM food of interest.
The Shared Assessment Process was developed through more than 10 years of collaboration, building on a long history of information-sharing between the two agencies, and broader international collaboration on GM foods. Additional information on the development of the Shared Assessment Process (and its initiative) can be found in the Organisation for Economic Co-operation and Development (OECD) consensus document: Considerations for Collaborative Work on the Safety Assessments of Foods and Feeds Derived from rDNA Plants (PDF 539KB).
Prior to implementing the Shared Assessment Process, Health Canada and FSANZ conducted two pilots to test the Process with either Health Canada or FSANZ as the primary assessor of a GM food. Learn more about the pilots.
The benefits of the Shared Assessment Process
During the course of the two pilot assessments, several benefits of the Shared Assessment Process were identified, including:
- Time and resource savings for the regulatory agencies
- Improved alignment and efficiency in the authorisation process for both Canada and Australia/New Zealand
- Faster authorisation times and/or cost savings for the applicant
Products suitable for the Shared Assessment Process
The current scope of the Shared Assessment Process is restricted to foods derived from genetically modified (GM) plants that require pre-market assessment under the regulatory frameworks of both agencies.
Furthermore, applications should meet the following criteria:
- Pre-market assessment and authorisation by both agencies is sought by the applicant within the same or similar time period.
- For applications which refer to information provided in a past application of another GM food, applicants should ensure that the GM food was previously assessed by both agencies such that each agency has access to the same information.
- Applicants provide identical information in their applications to each agency. Application format can be specific to the requirements of each agency.
- Applicants agree to inter-agency sharing and discussion of application-specific information, including confidential information, for the sole purpose of conducting the shared assessment.
How the Shared Assessment Process works
For the Shared Assessment Process, one agency acts as the primary assessor, while the other agency acts as the secondary assessor. For each GM food that undergoes a shared assessment, Health Canada and FSANZ will determine which role each agency will take on. As much as possible, the workload will be shared equitably between both agencies.
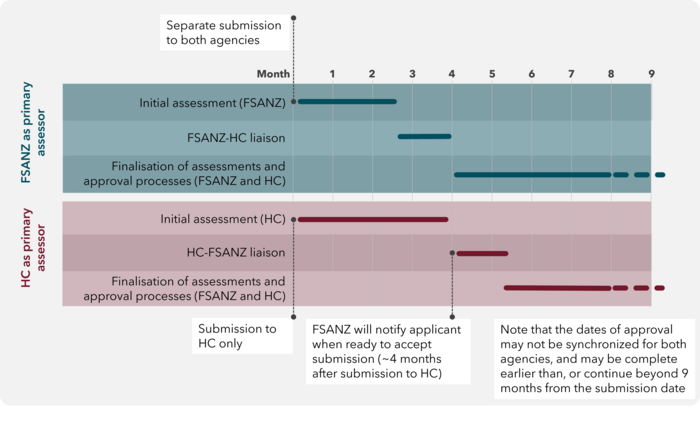
The Shared Assessment Process is outlined in Figure 1, and involves the following steps:
- Both agencies receive an application for the GM food that contains the same information. Refer to Figure 1 for submission timing.
- The application will be subject to the standard intake procedures of each agency. Should additional information be required before the application can be accepted for assessment, the primary assessor will obtain this information from the applicant and share it with the secondary assessor.
- Once an application is accepted for assessment, the primary assessor will conduct the initial assessment of the GM food. Depending on the complexity of the application, this step will take 2-4 months to complete. During this period, should any deficiencies in the application be identified, the primary assessor will interact with the applicant to obtain the necessary information for the assessment. Any additional information that is provided to the primary assessor per request should also be provided to the secondary assessor to ensure the identical content of the application with each agency.
- Once complete, the primary assessor will share its initial assessment with the secondary assessor to review. The primary and secondary assessors will then discuss the initial assessment to enable the secondary assessor to seek any clarifications from the primary assessor and highlight any additional aspects which should be included in the assessment. Following feedback from the secondary assessor the primary assessor will make the necessary revisions and provide a final version to the secondary assessor. The review process is expected to take approximately 3-4 weeks in total.
- Once the final version of the assessment is agreed, each agency will proceed with their respective post-assessment processes, leading to separate regulatory decisions regarding the GM food in question. During these processes, Health Canada and FSANZ will keep each other informed of their respective progress and anticipated authorisation timeline. Authorisation from each agency remain independent of each other.
How to submit a product for the Shared Assessment Process
To submit a product for the Shared Assessment Process, applicants should take the following steps:
- An applicant will inform both agencies of the intent to have their GM food undergo a shared assessment between the agencies. This can be done through an email to each agency or during a pre-submission consultation. It is important that an applicant explains how their product is suitable for the Shared Assessment Process, as well as their anticipated timeline for application.
- With the above information, Health Canada and FSANZ will determine, based on current capacities, who will be the primary and secondary assessor for the shared assessment. The applicant will be informed of this decision, as well as the projected timeline of the shared assessment including when to submit the application to each agency.
- If the applicant agrees to the terms of the shared assessment, an application for their GM food can be made through the regular channels of each agency at the agreed upon times. Application submission processes for FSANZ and Health Canada are available on their respective websites.
Further considerations
With the implementation of the Shared Assessment Process, Health Canada and FSANZ remain bound to all other regulatory, legal and/or policy requirements associated with their respective regulatory frameworks.
For example, Health Canada and the Canadian Food Inspection Agency (CFIA) exercise a ‘no split approvals’ policy regarding the authorisation of a GM plant for food and animal feed use, or for unconfined environmental release in Canada. While the shared assessment of a GM plant may result in a shorter assessment time with Health Canada, authorisation of that GM plant for food use will still be contingent on the completion of any required pre-market assessment conducted by the CFIA for the same GM plant.
Footnotes
- Defined as per Standard 1.5.2 of the Australia New Zealand Food Standards Code and Part B, Division 28 of the Canadian Food and Drug Regulations.